Step 1: Uselewis structure guidelines to draw the lewis structure of NO2.
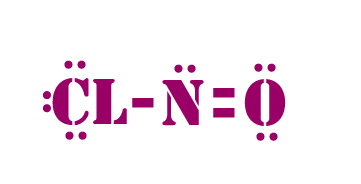
Step2: Apply VSEPR notation, A X E
A=Number of central atoms
X=Number of surrounding atoms
E= Number of lone pairs on central atom
For the above molecule VSEPR notation will be AX2E1
Step 3: Use VSEPR table to find the shape.
AX2E has angular/bent shape. So the shape of NO2 molecule is angular/bentl.
Download a copy of VSEPR shapes table here
Bond angle in NOCl
Bond angle in NO2 molecule is 120º due to repulsive force of lone pair of electrons on nitrogen.The representation is shown below.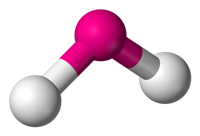
Image courtesy:wikipedia.org

