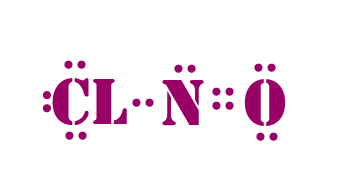Step 1: Find valence e- for all atoms. Add them together.
N:5
O:6
Cl:7
Total=18
Step2: Find octet e- for each atom and add them together.
C:8
O:8
Cl:8
Total=24
Step3: Gives you bonding e-. Subtract step 1 total from step 2
24-18=6e-
Step 4: Find number of bonds by diving the number in step 3 by 2(because each bond is made of 2 e-)
6e-/2= 3 bond pairs
Step 5: Find the number of nonbonding (lone pairs) e-. Subtract step 3 number from step 1.
18-6= 12e-=6 lone pairs
Use information from step 4 and 5 to draw the lewis structure.
According to electronegativity rule, Nitrogen has the least EN and needs three bonds to gain octet.So put nitrogen in the centre.
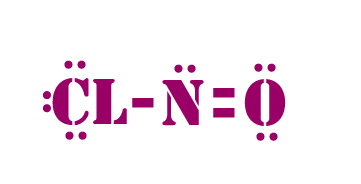
Lewis dot structure of NOCl
Alternatively a dot method can be used to draw the lewis structure.
Calculate the total valence electrons in the molecule.
N:5
O:6
Cl:7
Total=18
Put nitrogen in center and arrange oxygen and chlorine atoms on the sides.Arrange electrons until all elements get 8 electrons.