What is the Lewis Structure of PCl5?
What is the Lewis Structure of PCl5? Phosphorus pentachloride (PCl5) is a chemical compound composed of phosphorus and chlorine. It is a molecule which consists of five chlorine atoms attached to a central phosphorus atom. The P atom is hypervalent in this molecule, and the overall geometry is a trigonal bipyramidal arrangement of chlorine atoms.
What is this molecule?
Phosphorus pentachloride is primarily used as a chlorinating agent in organic synthesis. It can selectively replace hydroxyl groups (-OH) with chlorine atoms (-Cl) in organic compounds, a process known as chlorination. It is particularly useful in the production of various chlorinated compounds, such as acyl chlorides and alkyl chlorides. When phosphorus pentachloride reacts with water, it undergoes a vigorous reaction, producing hydrochloric acid (HCl) and phosphoric acid (H3PO4). This reaction is exothermic and releases heat. The compound is also employed as a catalyst in certain chemical reactions, such as the conversion of carboxylic acids to acid chlorides and the preparation of thionyl chloride (SOCl2).
Method 1: Step method to draw the Lewis structure of PCl5.
In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Here is a little flow chart of how we are going to do this:
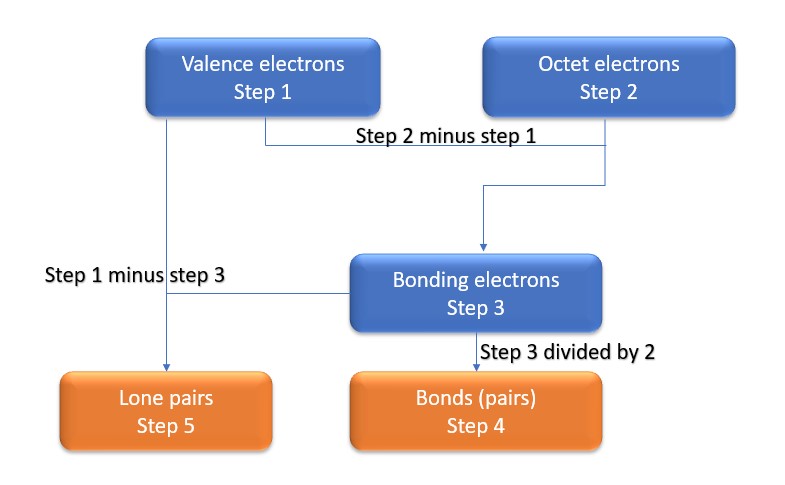
We will go through the steps below, but one thing to note here is that all the valence electrons (step 1) are either lone pairs OR bonding electrons. In other words…. Lone Pairs (Step 5) + Bonding electrons (Step 3) = Valence electrons (Step 1) . Let’s go through this example so we can see this a little more clearly.
Step 1: Find valence electrons for all atoms. This is determined by looking at which column on the periodic table the atom is in, ignoring the transition metals in the middle. Add the valence electrons for each atom together.
P: 1×5 = 5
Cl: 5×7 = 35
Total = 40 valence electrons
Step 2: Find octet electrons for each atom and add them together. Most atoms like 8 electrons to form an octet, however H is an exception to this.
P: 1x 10 = 10**
Cl: 5 x 8 = 40
Total = 50 “octet” electrons
NOTE: Phosphorous gets 10 valence electrons in this case since it must make 5 bonds with surrounding Cl atoms.
Step 3: Find the number of bonding electrons. Subtract the valence electrons (step 1) from the octet electrons (step 2). This gives the number of bonding electrons.
50-40=10 bonding electrons.
Step 4: Find number of bonds by diving the number of bonding electrons (step 3) by 2 because each bond is made of 2 e-
10 bonding electrons/2 = 5 bond pairs
Step 5: Find the number of nonbonding (lone pairs) electrons. Subtract bonding electrons (step 3) number from valence electrons (step 1).
40 valence – 10 bonding = 30 electrons = 15 lone pairs
Now, use the information from step 4 and 5 to draw the Lewis structures. Remembering too (this is important):
Phosphorus will have five bonds
Chlorine will have 1 bond and three lone pairs
[Note: For more information on the natural state of common atoms, see the linked post here.]
The phosphorus atom will be the central atom, and will connect to the five chlorine atoms. Each chlorine atom will have three lone pairs to complete the octet. Therefore the Lewis structure of PCl5 looks like this:

Another (easier) method to determine the Lewis structure of PCl5:
Alternatively a dot method can be used to draw the Lewis structure.
Calculate the total valence electrons in the molecule.
P: 1×5 = 5
Cl: 5×7 = 35
Total = 40 valence electrons
Now, treat the atoms and electrons like puzzle pieces. Put phosphorus in the center and arrange the chlorine atoms on the sides. Remember the natural state of each atom, as discussed above. [Phosphorus: 5 bonds, 0 LP; Chlorine: 1 bonds, 3 LP]. We will confirm each atom has their desired octet before we say this is our final answer.

Frequently asked questions:
Q: So what is the difference between the two methods?
A: In the first method, we are figuring out all of the lone pairs and bonds first, then placing those electrons and bonds on the atoms to form a molecule. In the puzzle method, we already have lone pairs and bonding electrons assigned to each atom, so all we need to do is push puzzle pieces together to get a molecule. In each method, we need to remember the “happy state” of each atom, ei hydrogen likes 1 bond and no lone pairs, uncharged carbon likes four bonds and no lone pairs ect.
Q: How can phosphorus have five bonds?
A: Overall, the ability of phosphorus to form compounds with five bonds is due to its ability to expand its valence shell by utilizing its vacant d orbitals, allowing for additional bonding opportunities. This is called hypervalency.
Now the super technical explanation: Phosphorus typically forms compounds with five bonds by utilizing its vacant d orbitals, which can accommodate additional electrons. In its ground state, phosphorus has three occupied orbitals in its valence shell (3s²3p³), leaving two vacant d orbitals available for bonding. The electronic configuration of phosphorus in PCl₅ involves hybridization of the 3s, 3p, and 3d orbitals to form five sp³d hybrid orbitals. Each of these hybrid orbitals overlaps with a 3p orbital of a chlorine atom, resulting in the formation of five sigma bonds. It’s important to note that the concept of five covalent bonds for phosphorus is a simplification used to describe the bonding in certain compounds. In reality, the electron density is spread out over the bonding regions, and the electron pairs are delocalized to some extent. Additionally, the actual nature of bonding can be described using molecular orbital theory, where the orbitals from multiple atoms combine to form molecular orbitals.
And now some video:
This is a quick video we put together that visually demonstrates the two methods for Lewis structure and Lewis dot problems.
And finally, the Lewis structure study guide:
Here it is, this is our one-page guide to Lewis Dot and Lewis Structures:
