What is the Lewis Structure of O2?
What is the Lewis Structure of O2? The Lewis structure of diatomic oxygen has oxygen connected to oxygen by a double bond, with two lone pairs on each oxygen atom.
What is this molecule and what is it used for?
O2, molecular oxygen or diatomic oxygen, is a molecule you know pretty well already from every day life, cause it keeps us alive. O2 is a colorless, odorless, and tasteless gas in its elemental form and makes up about 21% of our atmosphere today (fun fact: in prehistoric times, it used to be way more, which is one of the reasons animals were so big back then). While it does have industrial and medical uses, it also is an important part of our atmospheric ozone, as lightning can cause the following reaction: 3O2 -> 2O3
Method 1: Step method to draw the Lewis structure of O2.
In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Here is a little flow chart of how we are going to do this:
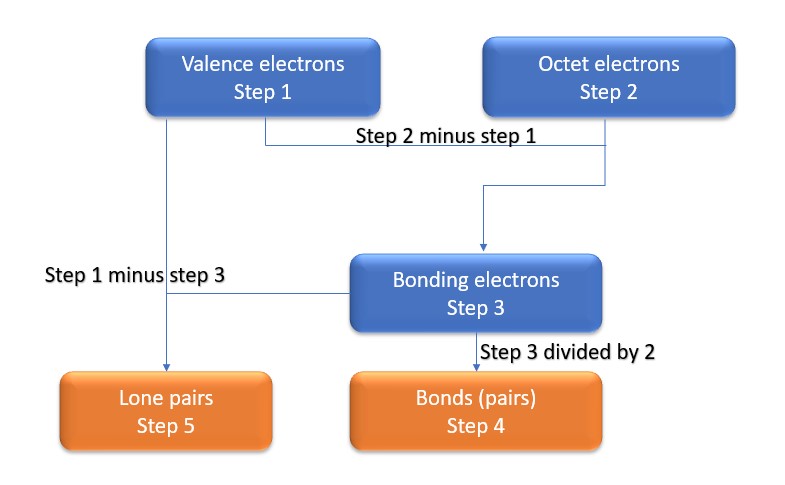
We will go through the steps below, but one thing to note here is that all the valence electrons (step 1) are either lone pairs OR bonding electrons. In other words…. Lone Pairs (Step 5) + Bonding electrons (Step 3) = Valence electrons (Step 1) . Let’s go through this example so we can see this a little more clearly.
Step 1: Find valence e– in all atoms. Add them together.
O=6 x 2
Total=12
Step2: Find octet e- for each atom and add them together.
O=8 x 2
Total=16
Step3: Gives you bonding e-. Subtract step 1 total from step 2
16-12=4e-
Step 4: Find number of bonds by diving the number in step 3 by 2(because each bond is made of 2 e-)
4e-/2= 2 bond pairs
Step 5: Find the number of nonbonding (lone pairs) e-. Subtract step 3 number from step 1.
12-4= 8e-=4 lone pairs
Now, use the information from step 4 and 5 to draw the Lewis structures. Remembering too (this is important):
Neutral oxygen has two bond and two lone pairs
[Note: For more information on the natural state of common atoms, see the linked post here.]
We connect the oxygen and oxygen because they are the only two atoms in the molecule. We place the two bonds from step 4 between the atoms and then evenly distribute the lone pairs.
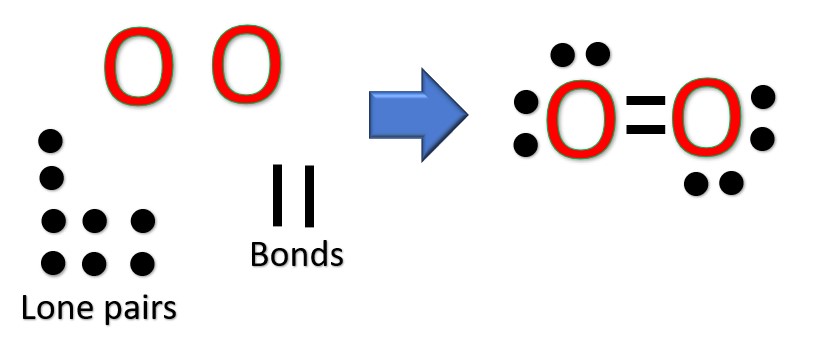
Another (easier) method to determine the Lewis structure of O2:
Alternatively a dot method can be used to draw the Lewis structure.
Calculate the total valence electrons in the molecule.
O : 2×6 = 6
Total = 12 valence electrons
Now, treat the atoms and electrons like puzzle pieces. We have to rearrange electrons to make sure everyone has an octet. To do this, make single bonds between the atoms, then take lone electrons and turn them into bonds to make sure each atom has an octet. After we have done this, we see there is a double bond between the atoms and two lone pairs on each.

Frequently asked questions:
Q: So what is the difference between the two methods?
A: In the first method, we are figuring out all of the lone pairs and bonds first, then placing those electrons and bonds on the atoms to form a molecule. In the puzzle method, we already have lone pairs and bonding electrons assigned to each atom, so all we need to do is push puzzle pieces together to get a molecule. In each method, we need to remember the “happy state” of each atom, ei hydrogen likes 1 bond and no lone pairs, uncharged carbon likes four bonds and no lone pairs ect.
And now some video:
This is a quick video we put together that visually demonstrates the two methods for Lewis structure and Lewis dot problems.
And a video specifically on the Lewis Structure of CO:
And finally, the Lewis structure study guide:
Here it is, this is our one-page guide to Lewis Dot and Lewis Structures:
