Step 1: Use lewis structure guidelines to draw the lewis structure of BCl3.

Step2: Apply VSEPR notation, A X E
A=Number of central atoms
X=Number of surrounding atoms
E= Number of lone pairs on central atom
For the above molecule VSEPR notation will be AX2E0
Step 3: Use VSEPR table to find the shape.
AX2 has linear shape. So the shape of BeF2 molecule is linear.
Download a copy of VSEPR shapes table here
Bond angle in BeF2
Bond angle of F-Be-F covalent bond in this molecule is 180º.The representation is shown below.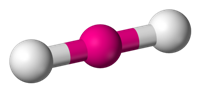
Image courtesy:wikipedia.com

