Step 1: Find valence e- in all atoms. Add them together.
I:7
F:4x7=28
Charge=1
Total=36
Step2: Find octet e- for each atom and add them together.
I:8
F:4x8=32
Total=40
Step3: Subtract step 1 total from step 2.This step gives you number of bonding e-.
40-36=4e-
Step 4: Find number of bonds by diving the number in step 3 by 2(because each bond is made of 2 e-)
4e-/2= 2 bond pairs.Since 2 bond pairs are not enough to make 4 bonds with central atom, adjust the octet of central atom to 12 electrons.
Step2: Find octet e- for each atom and add them together.
I:12
F:4x8=32
Total=44
Step3(revised): Subtract step 1 total from step 2.This step gives you number of bonding e-.
44-36=8e-
Step 5: Find the number of nonbonding (lone pairs) e-. Subtract step 3 number from step 1.
36-8= 28e-=14 lone pairs
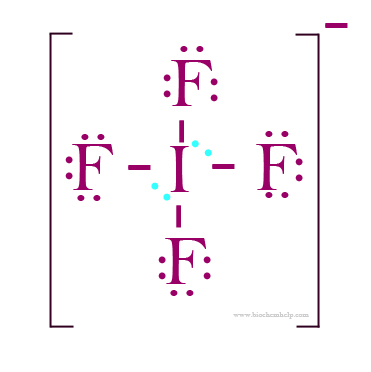
Use information from step 4 and 5 to draw the lewis structure.
Put atom(s) with lowest electro negativity in the center. Arrange the remaining atoms around it. Finally put the bond pairs and lone pairs of electrons on the atoms.