What is resonance?
What is resonance (in organic chemistry) in 2022? In one sentence, it is the concept where electrons (bonds) are delocalized over three or more atoms which cannot be depicted with one simple Lewis structure.
Resonance is one of those issues that you will have to deal with for both semester I & II of organic chemistry. It is much better to have a solid understanding of it now, rather than have to worry about it later. The basic goal of resonance structures is to show that molecules can move electrons and charges onto different atoms on the molecule. Resonance generally makes a molecule more stable because the charge (or bond) is now delocalized and not “forced” onto an atom that might not want it. More on this is discussed below!
What is the resonance effect and why care?
The best way to discuss this concept is to talk about the resonance effect. This concept says that the more resonance structures you that can be formed means more stability in that molecular compound. Said another way: a carbocation structure with no resonance structures will be less stable than one with multiple resonance structures.
Sometimes, molecules can be represented with more than one Lewis/resonance structure, where the only difference is the location of pi electrons. Electrons in sigma bonds have a fixed location; these are called localized and never move. Conversely, pi electrons are referred to as delocalized, because they can be easily moved around. Together, these Lewis diagrams are then known as resonance structures or resonance contributors or resonance canonicals. The actual molecule has characteristics of each of the parts, and can represented as a resonance hybrid (which you can think of as a cross-breed). Resonance hybrids are a more accurate way to think about resonance structures, because it is more like what the structure looks like in nature.
Some Rules of Resonance
Below are some handy rules of resonance. If you learn these and think about them when tackling different problems, you will be able to handle whatever is thrown at you.
1) Know each atom’s “natural state”. We talk about this in a different post on atoms’ natural state. You need to recognize what each atom we deal with generally looks like, in an uncharged state. This will help you to construct the Lewis Dot structure on which you will base your structures. Remember that halogens and hydrogens are always terminal, meaning that are at the end of the molecule and only have one bond, and therefore, they will not (generally) participate.

2) Atom positions will not change. Once you have determined that an atom is bonded to another atom, that order will not change in a resonance structure. If they do change, it is no longer a resonance structure, but is now a constitutional isomer or a tautomer. Here is a nice post on constitutional isomers to check out. This is important and instructors will try to trick you on it. Don’t fall for it.

3) Check the structure you have created to make sure that it follows the octet rule. This will become much easier once you have a better handle on the “natural state” of atoms. If you violate the octet rule, you need to go back and check to make sure you didn’t make a mistake. Don’t remember the octet rule? It is where each atom needs to have eight electrons in its outer most shell to be most stable. This is important to go back and review if you don’t remember it. Here is a good post on it.

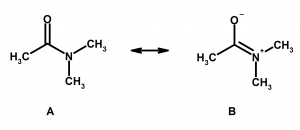
4) When two or more structures can be drawn, the one with the fewest total charges is the most stable. In the example below, A is more stable than B. Multiple charges on atoms CAN exist (such as a zwitterion), but this is usually seen when there is an acid and a base on the same molecule, not in this case. Once you see more organic chemistry, this will just become intuitive to you.
5) When two or more structures can be drawn, the more stable has the negative charge on the more electronegative atom. Hopefully, this makes sense to you. The more electronegative atom is better able to accommodate a negative charge, therefore the more stable structure will put the negative charge on the atom in which it will be more stable. In the example below, A is more stable than B.

6) In the end, each resonance structure should have the same overall charge and total number of electrons (bonds + lone pairs) as when you started. If you started with a negative overall charge, you should end with one. If it does not, you most likely made a mistake somewhere.
7) Resonance affect the length of a bond between two atoms. We have another post on this topic, which we called resonance part 2. This is far less intuitive but makes more sense when you think of it in terms of resonance hybrids. Basically, resonance can make two bond lengths equal even though we might draw it as something different on paper, like we show below here:
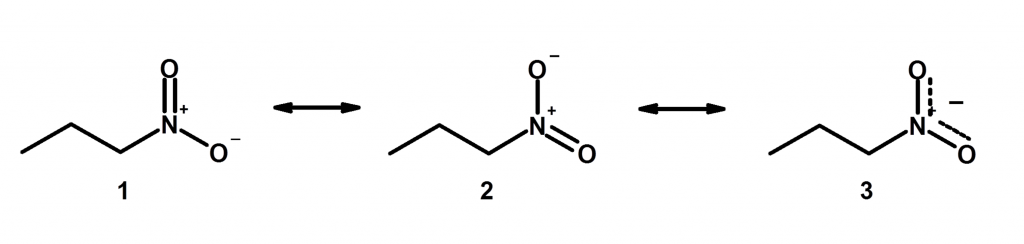
Take Home Message: Resonance is like telemarketers. They are never going to go away, so you need to learn them well.

Reference: resonance

