
WARNING: Solvent-Separated Ion Pair concept is somewhat advanced. It can be simply explained by your professor might not require you to have knowledge of it. Only moderate to severe organic chemistry nerds should proceed.
Hi everybody, just wanted to give out a quick tip when looking at SN1 reactions. Many students believe that SN1 reactions give totally racemic products, but this is not actually the case. The amount of loss of chirality depends on the leaving group, the solvent and the nucleophile.
We all know that the SN1 reaction proceeds through a carbocation intermediate, which cannot hold the stereochemistry that the carbon atom previously possessed. (In other words, if you start with a chiral carbon and go through a cation, you will have a trigonal, achiral cation) . Shown below is a very simple example of an SN1 reaction between t-butyl chloride and sodium cyanide.
However your starting material does not actually have the same chance of being attacked by the cyanide from either side. Many times, the leaving group can come back to the cation on the same side. This partially blocks one face of the cation. The net result is that your carbocation CANNOT be equally attacked from both sides. Below is a stages that the leaving group can go through as it takes off.
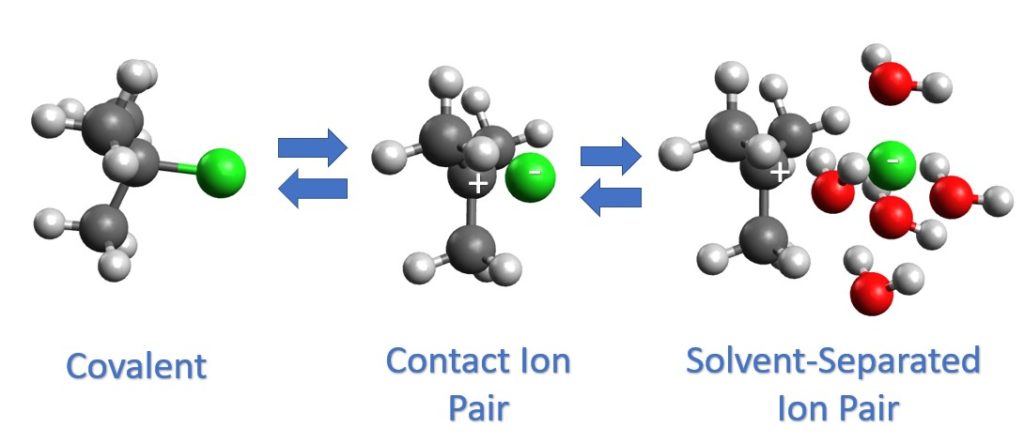
In this example, our starting material (which will become the electrophile in the SN1 reaction) is t-butyl chloride. The far left structure is the starting material with a normal covalent bond. In the middle is the contact ion pair, which is also called the intimate ion pair. This is an ionic bond, which the chlorine is a full anion, but has not gone far from the carbocation. In the contact ion pair, the solvent has not come between the two charges and the chloride ion has the highest likelihood of blocking nucleophile attack from that side. At any time, the chloride ion can re-attach to the carbocation and become a covalent bond, as shown by the double arrows. From a contact ion pair the chlorine can get farther from the carbocation and become a solvent-separated ion pair. Here the chloride ion still has some affect on which face the nucleophile attacks from, but less influence than if it is was a contact ion pair.
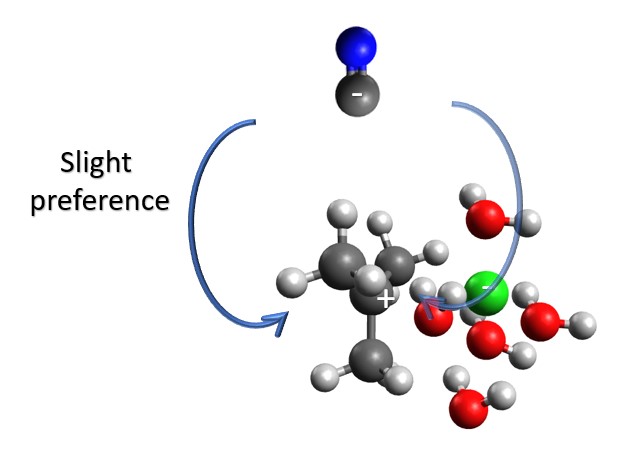
When the nucleophile attacks, it can come in from either face of carbocation, but not in equal ratios, as the chlorine is still blocking one face slightly more than the other. While the preference for the unblocked face may be small, you can see the difference in some reactions. This means that the SN1 reaction will lose some (or a lot of) chirality, but will not usually become totally racemic.
Good luck on those exams and happy reacting.

