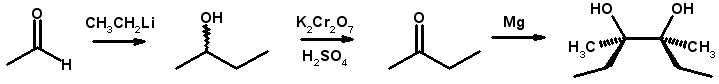
Some of your professors will try to sneak this one in on you during an exam: Your professor will ask you to synthesize the symmetric diol shown below and you will either not know how or you will come up with a convoluted, difficult or wrong answer. JUST REMEMBER: Symmetric diols came from the Pinacol reaction.
While not obvious, there is a very simple way to create a symmetric diol using the Pinacol reaction. Using a number of reducing agents, ketones and aldehydes can be coupled with themselves to form a symmetric diol, as shown below:
The reaction is also called the Pinacol Coupling Reaction and can be used on most aldehydes and ketones, but not on acid halides or carboxylic acids. Now to address the original synthetic problem above:
The first step is reaction of the reaction of ethyl lithium with ethanal to form 2-butanol. This is then oxidized to 2-butanone using Jones’ oxidation. Now, under Pinacol Coupling conditions, 2-butanone is then reacted with itself using magnesium to form the final product.
Take Home Message: Symmetric 1,2 diols came from the Pinacol reaction.
For more organic chemistry help, go to organic chemistry




Hello Dear
please send me books about INORGANIC CHEM.& Books about symmetric in Inorganic chem.
Thanks a lot
s.m.shishvan
Late to the party, but is the cis isomer the only one formed from butanone?