Newman projection practice problems
When looking at Newman projection practice problems, there are a few areas that we need to make sure that we emphasize so you see the entire range of problems that might appear on an exam. First however, we should do a brief review on Newman projections. To see a full review of Newman projections, see this previous blog post. Briefly, Newman projections are a way to view simple organic molecules by looking at down the axis between two carbon atoms. We show the carbon atoms as little balls and the substituents as sticks coming off those balls at 120 degrees away from each other. There are three primary configurations a Newman projection can be in: staggered, eclipsed, and gauche. Below you can see examples of each one of these.
Now that we know what a Newman projection is, let’s look at what the most common types of questions you will see are:
Problem #1: Can you draw a Newman projection? Draw the Newman projection of butane.

This is the most basic of Newman projection practice problems. All you have to do to master these problems, is find out where the axis of interest is. Please note, this is much different than the axis of Evil.
Generally, the main axis, or axis of interest, or carbon-carbon bond of interest will be a central carbon bond in your molecule. For example, in butane the most common axis for Newman projection is the C2-C3 axis. Of course, you can draw a Newman projection down any bond axis, but the C2-C3 axis is the most beneficial to view in a Newman projection.
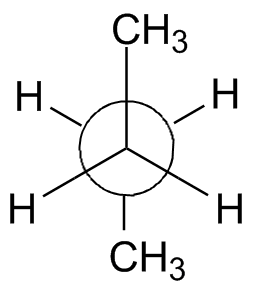
Below are some more questions to help you practice this.

Problem #2: Do you understand relative energies? Which are the lowest and highest energy confirmations of a Newman projection?
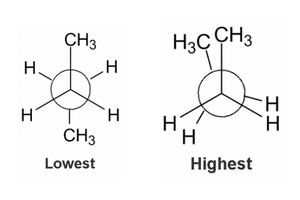
If we take what we learned above, with respect to how to draw a Newman structure and the types of conformations that exist, this problem becomes much easier. For the purposes of undergraduate organic chemistry, 98% of the time the lowest energy structure is going to be a staggered structure where the largest groups are 180 degrees away from each other. This is the lowest energy conformation because there is limited torsional and steric strain. We will go into torsional and steric strain more below. Try these practice problems to see if you can come up with the lowest energy conformation of these Newman projections.
Problem #3: Do you understand strain? Is there torsional or steric strain?
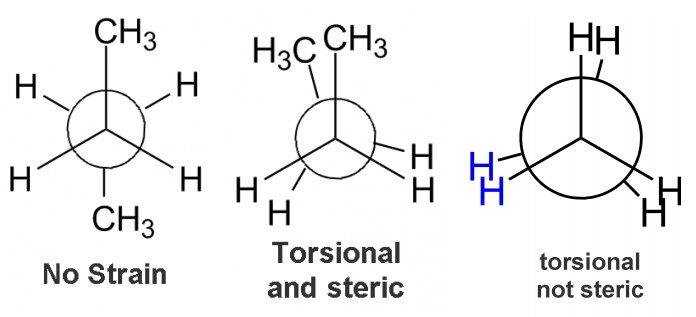
There are two types of strain that can be seen using a Newman projection. The first is steric strain. This is much more common in other types of organic chemistry problems, and occurs when two large groups are near each other. With respect to Newman projections, anytime two groups that are a methyl size or larger are near each other there is steric strain. Hydrogen-hydrogen interactions do not really lead to steric strain like a methyl-methyl interaction will.The second type of strain is torsional strain, which occurs when bonds overlap. For the purposes of Newman projections, anytime we have an eclipse conformation there will be some torsional strain. Armed with this knowledge, let’s look at some more problems.
Practice problem #4: Do you understand relative energies? Draw the energy diagram for a Newman projection:
Energy diagrams show the relative energy of a molecule compared to rotation about the axis of interest. Generally, these start at 0 degrees and rotate through the entire molecule. This can then be graphed showing which parts and bond angles about the axis of interest are more or less stable. Because this diagram is relative, we will make some educated guesses when we draw it out. To make one of these energy diagrams, we have to have a good idea ahead of time of what the lowest and highest energy conformations are. To do it thoroughly we are going to walk through the conformation and check the relative energy every 60°. Since there are 360 degrees in a full rotation, that means we will have six different energy points to plot on our diagram per molecule. Here is a good example of what one of those will look like.
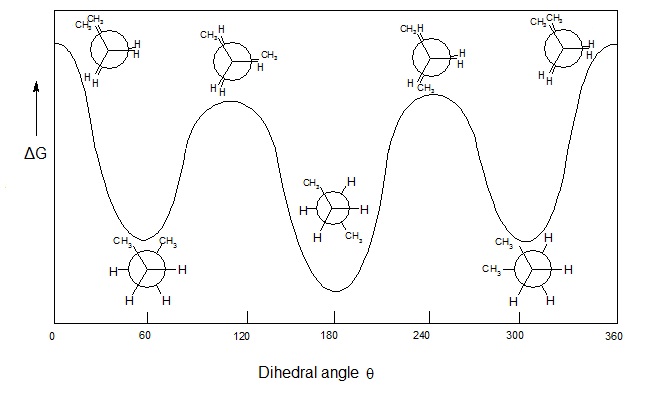
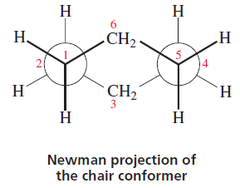
Newman projection practice problems #5: What about advanced Newman projections? Draw cyclohexane as a Newman projection.
Students should realize the Newman projections are not just confined to linear molecules. Newman projections can be used in cyclic molecules also. Some professors like to test this concept using cyclohexane. With a little bit of practice, this can be done relatively simply. The main thing to remember is that in cyclohexane’s Newman projection there are two axes of interest. Could you do this with three axises? You bet.