What is the Lewis Structure of BeF2?
What is the Lewis Structure of BeF2? The Lewis structure has beryllium as the central atom, with a fluorine atom on each side. Each fluorine atom has three lone pairs while the beryllium atom has no lone pairs.
What is this molecule?
BeF2, also known as beryllium fluoride, is a chemical compound primarily used in various industrial applications, to include use as a fluxing agent in the production of ceramics and glass, use as an electrolyte in batteries, and also as a chemical catalyst in certain chemical reactions.
Method 1: Step method to draw the Lewis structure of BeF2.
In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Here is a little flow chart of how we are going to do this:
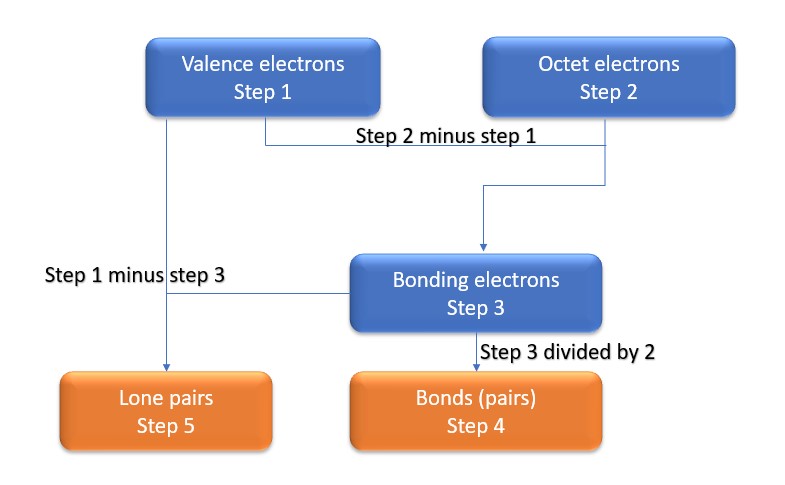
We will go through the steps below, but one thing to note here is that all the valence electrons (step 1) are either lone pairs OR bonding electrons. In other words…. Lone Pairs (Step 5) + Bonding electrons (Step 3) = Valence electrons (Step 1) . Let’s go through this example so we can see this a little more clearly.
Step 1: Find valence electrons for all atoms. This is determined by looking at which column on the periodic table the atom is in, ignoring the transition metals in the middle. Add the valence electrons for each atom together.
Be : 1×2 = 2
F : 2×7 = 14
Total = 16 valence electrons
Step 2: Find octet electrons for each atom and add them together. Most atoms like 8 electrons to form an octet, but there are exceptions and Be is one of those exceptions, as it only needs four electrons for an octet.
Be: 1×4 = 4
F: 2×8 = 16
Total = 20 “octet” electrons
Step 3: Find the number of bonding electrons. Subtract the valence electrons (step 1) from the octet electrons (step 2). This gives the number of bonding electrons.
20-16=4 bonding electrons.
Step 4: Find number of bonds by diving the number of bonding electrons (step 3) by 2 because each bond is made of 2 e-
4 bonding electrons/2 = 2 bond pairs
Step 5: Find the number of nonbonding (lone pairs) electrons. Subtract bonding electrons (step 3) number from valence electrons (step 1).
16 valence -4 bonding = 12 electrons = 6 lone pair
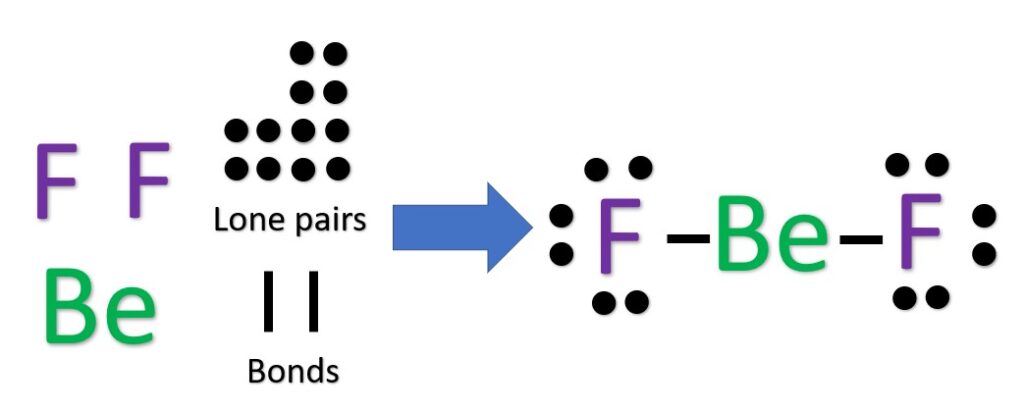
Now, use the information from step 4 and 5 to draw the Lewis structures. Remembering too (this is important):
Beryllium likes 2 bonds and no lone pairs
Fluorine has one bond and three lone pairs
[Note: For more information on the natural state of common atoms, see the linked post here.]
The Be atom will be our central atom because it is the most electropositive. We then place the fluorine atoms around it, creating one bond to each F atom. Behold, BF2 Lewis dot structure.
Another (easier) method to determine the Lewis structure of BeF2:
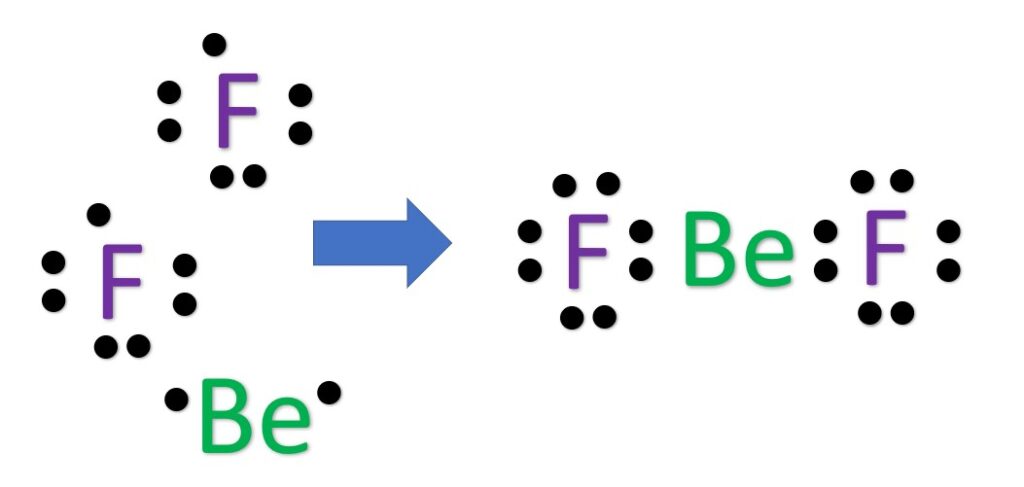
Alternatively a dot method can be used to draw the Lewis structure.
Calculate the total valence electrons in the molecule.
Be : 1×2 = 2
F : 2×7 = 14
Total = 16 valence electrons
Now, treat the atoms and electrons like puzzle pieces. Put beryllium in the center and arrange the fluorine atoms on the sides. Remember the natural state of each atom, as discussed above. [F: 1 bonds, 3 LP; Be: 2 bonds, 0 LP; ] Also, make sure sure each atom has an octet, which in the case of beryllium is only four electrons.
Frequently asked questions:
Q: So what is the difference between the two methods?
A: In the first method, we are figuring out all of the lone pairs and bonds first, then placing those electrons and bonds on the atoms to form a molecule. In the puzzle method, we already have lone pairs and bonding electrons assigned to each atom, so all we need to do is push puzzle pieces together to get a molecule. In each method, we need to remember the “happy state” of each atom, ei hydrogen likes 1 bond and no lone pairs, uncharged carbon likes four bonds and no lone pairs ect.
Q: Why does beryllium only need four electrons for an octet?
A: Beryllium, with an atomic number of 4, belongs to Group 2 of the periodic table. In terms of its electronic configuration, it only has four total electrons, with two electrons in its innermost shell (1s2) and two electrons in its outermost shell (2s2). Atoms become stable when the outermost shell is full, which is usually 8 electrons. Yet, in the case of beryllium, it is an exception to the octet rule because it is stable with only four electrons in its outermost shell.
Because Be has a small atomic radius, the 2s orbital, which is the outermost orbital for beryllium, is closer to the nucleus compared to higher energy orbitals from in other atoms. As a result, the outermost electrons are strongly attracted to the nucleus and held tightly. The energy required to either gain four more electrons or lose the existing two electrons from the 2s orbital is significantly high, making it energetically unfavorable for beryllium to achieve an octet. Instead, beryllium tends to form compounds by sharing its two valence electrons with other elements, resulting in a stable electron configuration with only four electrons in its outermost shell. Those four electrons are the two it brings to the party itself, from its 2s orbital, and the two electrons it picks up from other atoms to form two bonds.
And now some video:
This is a quick video we put together that visually demonstrates the two methods for Lewis structure and Lewis dot problems.
And finally, the Lewis structure study guide:
Here it is, this is our one-page guide to Lewis Dot and Lewis Structures:

