Electrophilic Addition: 2022 Edition
Electrophilic addition: Just like in football, it is easy to say that one of the players is the most important one in the game. But they all play an important part, and electrophiles are one of those important parts.

While many (nerdy) organic chemists could have a robust debate over a pint as to which of the compound class is most valuable in the reaction, we are going to treat them all as important. In its most basic form, they are all essential in some way or another to the reaction’s success. Hence, we are going to start with acids and discuss all of the compound classes one by one.
Electrophiles are one of the two most important reactants in organic chemistry (spoiler alert: the other is a nucleophile, check out our blog post on those here). As we have discussed previously on this blog, organic chemistry reactions are all about the flow of electrons, and electrophiles are the ones who want those electrons. When you think of the word “electrophile” you should think of the Greek word “Philos” which means “to love”. Therefore, an electrophilic species is one that loves electrons. Easy enough, right? Since opposites attract, and the electrophile loves electrons, then it must be that the electrophile is positively charged. Most often, you will see this abbreviated as “E+”.

So the question now becomes: what make an atom a good electrophile and how do we spot it? Since we know the electrophiles want to electrons, the first clue that something is electrophilic is that it has a positive charge. The second clue is if we can place a positive charge somewhere on the atom via resonance and that it has an empty orbital (positive charge or metal with an empty orbital) or can get an empty orbital by kicking off a leaving group. Below are some common classes of electrophiles you will see frequently in your course:

– In example A, a carbonyl is shown. We know that the carbon of the carbonyl is electrophilic because we can place a positive charge on it via resonance. This means that a nucleophile will attack the carbonyl at this carbon atom. [Fun fact: the oxygen of the carbonyl is actually way less nucleophilic than one might think]
-In example B, we show diatomic chlorine. Diatomic halogen molecules (Cl2, Br2, I2) are electrophilic because the bond between the halogen atoms as polarizable, meaning that the electrons can reside on either atom at any time, making one of the atoms more electrophilic than the other at any one given time. Think of it as the electron density jumping back and forth from one chlorine atom to another, making one of them more electrophilic for just an instant.
-In example C, we see that alkyl halides are also electrophilic because of a polarizable bond between the carbon and the chlorine atoms. Unlike example B, example C is a permanent dipole.
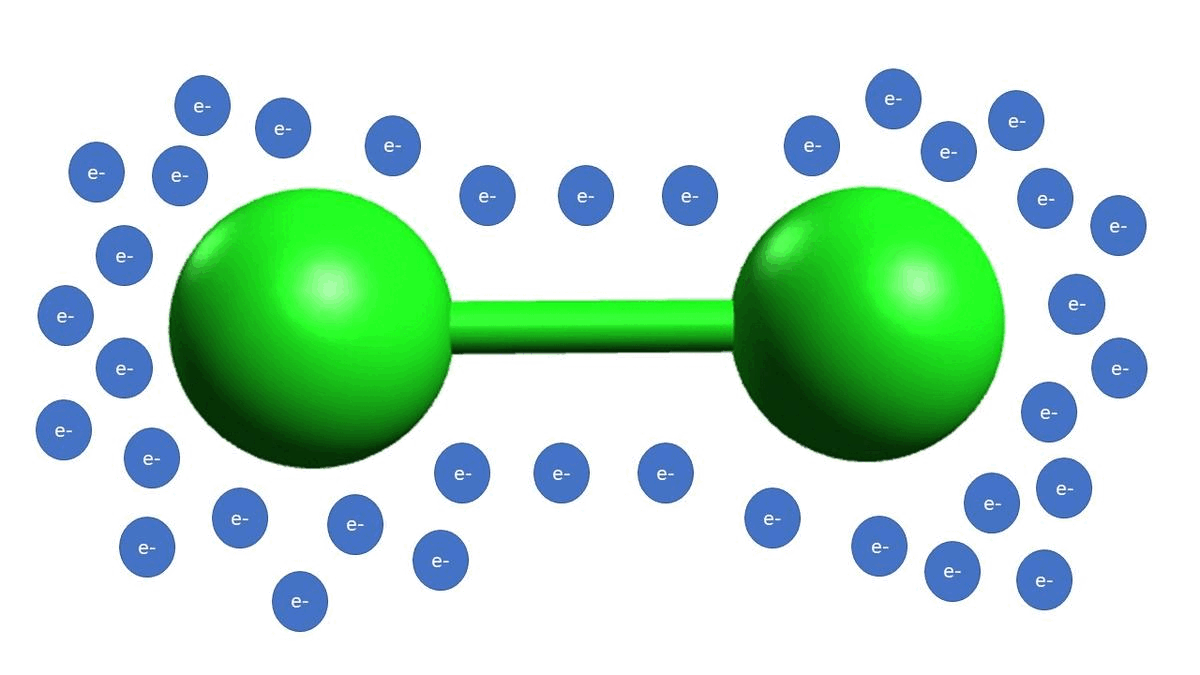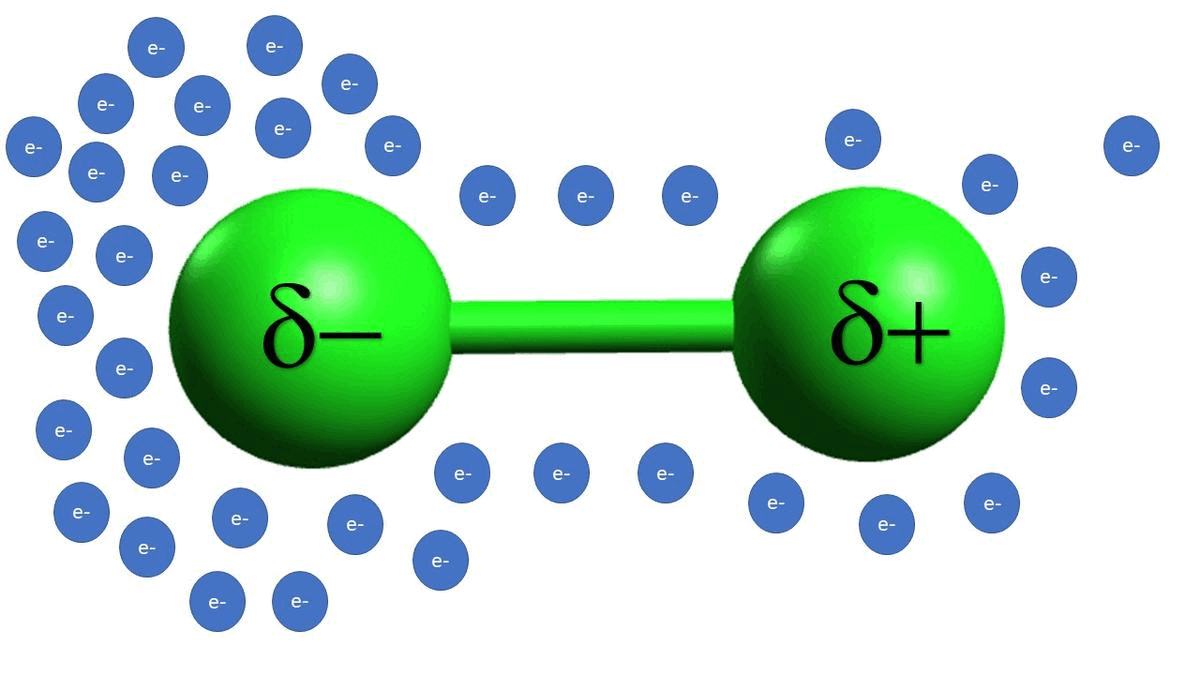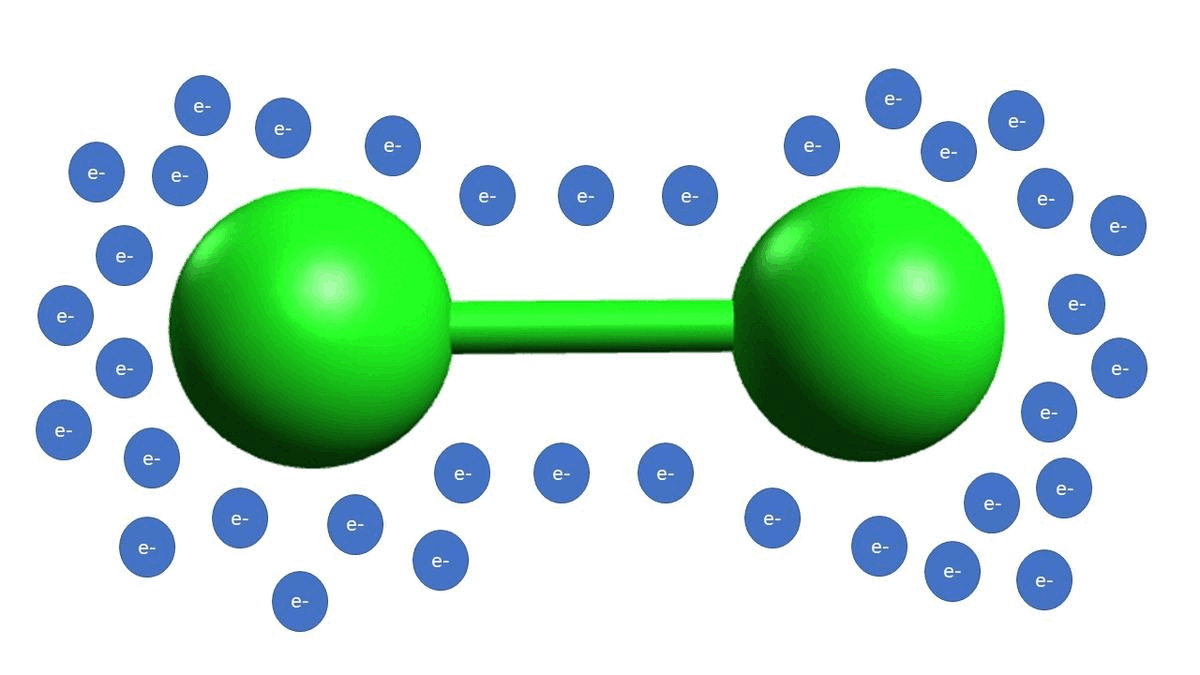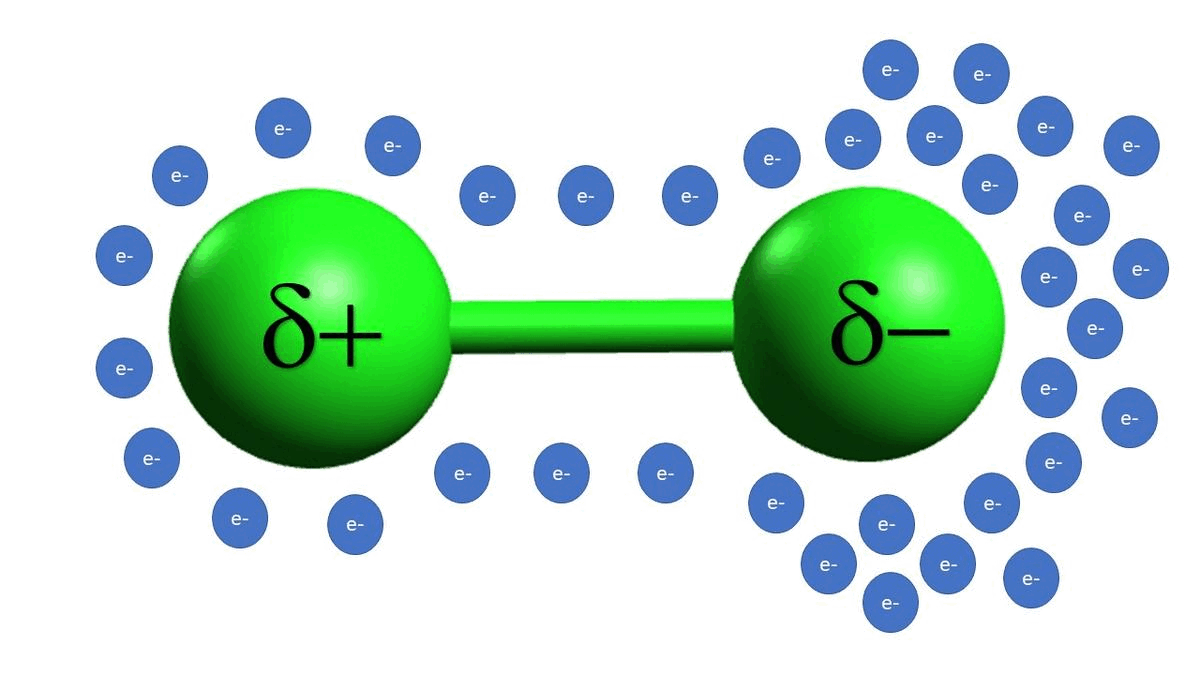

-Example D is an example of a strong acid completely disassociating, which gives off a proton as the electrophilic species.
-Finally in example E, we see it you can create an electrophile from a non-electrophilic molecule. Here we have reacted nitric acid with sulfuric acid to form the nitronium ion, which is highly electrophilic.

Electrophiles are also Lewis Acids. Lewis acids accept electron density, because they are electron deficient. The reagent acting as the Lewis Acid can suck electron density from the electrophile, making even more electron deficient and therefore even more reactive. Crazy, right?

Take home points on electrophiles:
1) They want electrons, meaning they are electron deficient, in order to form a new bond.
2) They are attacked by nucleophiles.
3) They are positively charged (or have a partial positive), polar and/or polarizable.
4) They become even better electrophiles in the presence of Lewis acids.
Would you like to learn about the nucleophiles that will attack these electrophiles? Please go to strong nucleophiles to get a good flavor of those.
And now, electrophilic addition reactions:
For more help with organic chemistry, please see organic chemistry help
Reference: Electrophiles


Pingback: Know your strong nucleophiles « Organic Chemistry made easy