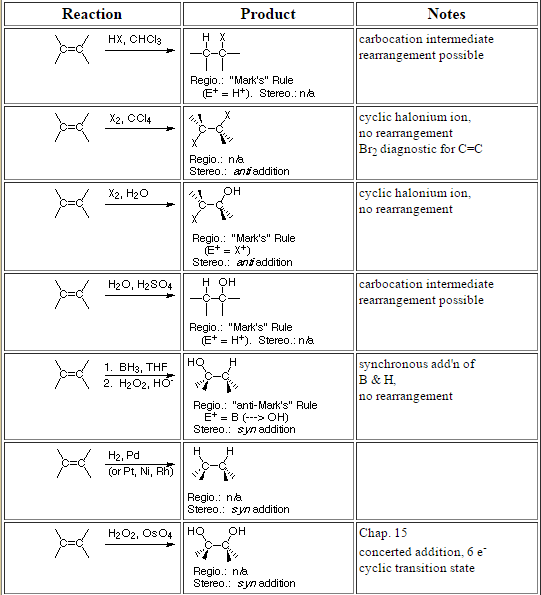
Alkene reactions
Alkene reactions are great because a double bond in organic chemistry gives you all sorts of way to add on to the molecule and create a bunch of different products. The chemistry works because there is a ton of electron density within the pi bonds of the double bond. Since sigma bonds are stronger than pi bonds, double bonds tend to react to convert the double bond into sigma bonds. In many cases, the mechanisms of these reactions will proceed through a carbocation mechanism, which means we should have a discussion about Markovnikov’s rule. But that is another post you should look at.
Most of these reactions fall under the umbrella of electrophilic reactions of alkenes. This is because, as stated above, there is electron density in the pi bond, which means it will react with electrophiles. Of note, most of these alkene reactions are second semester organic chemistry reactions. If you are in first semester and have not seen these reactions before, do not panic. Remember that first semester organic chemistry is heavy on concepts but light on reactions. It should not come as a surprise if you have not seen these before. First semester students should look over the chart below if they are interested, but you probably won’t see most of these on an exam anytime soon.
We also found a guide online that looks pretty good, click here to download it—> alkene guide
Here are some of our favorite ones below, give it a look.