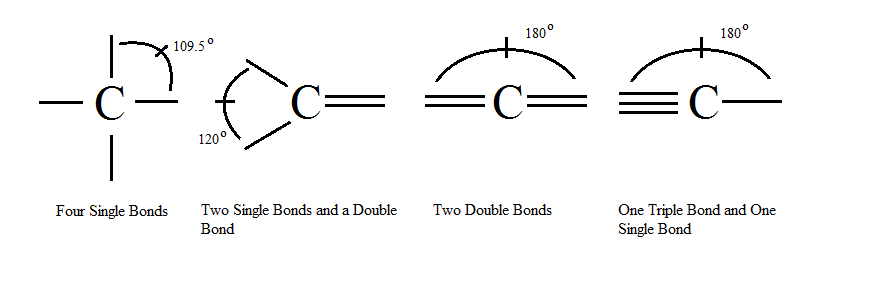If you haven’t noticed by now, carbon is the most important atom in the organic chemistry world. It is all about carbon chemistry.
Here’s a hint: if it doesn’t have carbon in it, it is most likely not an organic molecule. Carbon is the basis of everything in this class. In some cases carbon will be a nucleophile, and in others it will be an electrophile. If you’re just starting out, there are some things you should know about carbon that will make your life a lot easier through your organic chemistry class.
- Carbon is the most important atom in the organic chemistry world. I think we have sufficiently emphasized this point now.
- The sp3 carbon atom is a tetrahedral molecule with bond angles at exactly 109.5 degrees.
- Carbon atoms can be hybridized in three separate ways. sp, sp2 and sp3. sp3 hybridized carbons are seen in alkanes. sp2 hybridized carbons are seen in alkenes, and sp hybridized carbons are seen in alkynes. See below for a good picture of the bond angles for each.
- Carbon usually has four bonds to it. It can sometimes have three bond, and in rare circumstances even have two bonds. It will never never never never never have 5 bonds. Did I say never enough times? Seriously, don’t ever put 5 bonds to carbon please.
- Chains of carbon are called hydrocarbons and really only do two things. They are solvents, and they can be burned. That’s really about it. This is because there is not a huge difference in electronegativity between carbon and hydrogen. Therefore it is not a polar bond, which means it doesn’t have polarized electrons which facilitate chemical reactions. This makes hydrocarbons very boring and non-reactive. Nonpolar, boring nonreactive molecules are great nonpolar solvents. But other than that they really don’t do much. There are ways to activate the C-H bond to do chemistry, but most of those are beyond the scope of undergraduate organic chemistry. The exception is free radical halogenation of an alkane.