Q: What is a meso compound?
Q: Why should we care about them in our organic chemistry class?
We care because this is an easy place for a professor to try to trip you up.
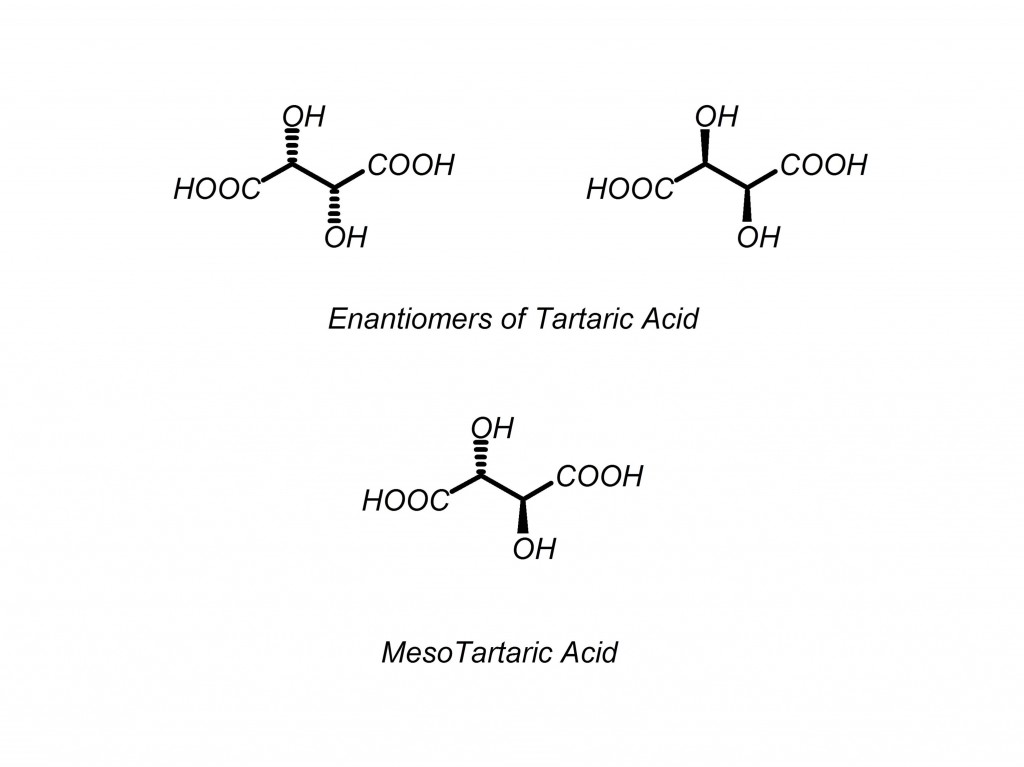
What is a meso compound? Meso compounds are molecules that have multiple stereocenters but are superimposable on their mirror images. Meso compounds do not have an enantiomer but do have a plane of symmetry. Meso compounds are not optically active but are three-dimensionally shaped. They have dashes/wedges on stereocenters, but they are not chiral.
Test your molecule: flip all of the chiral centers and check to see if it is the same molecule as you had before you flipped the centers. If the molecule is the same, it is meso. If it is not superimposible on its mirror image, it is chiral. Below is a good example of this.
Question: How many forms of tartaric acid are there?
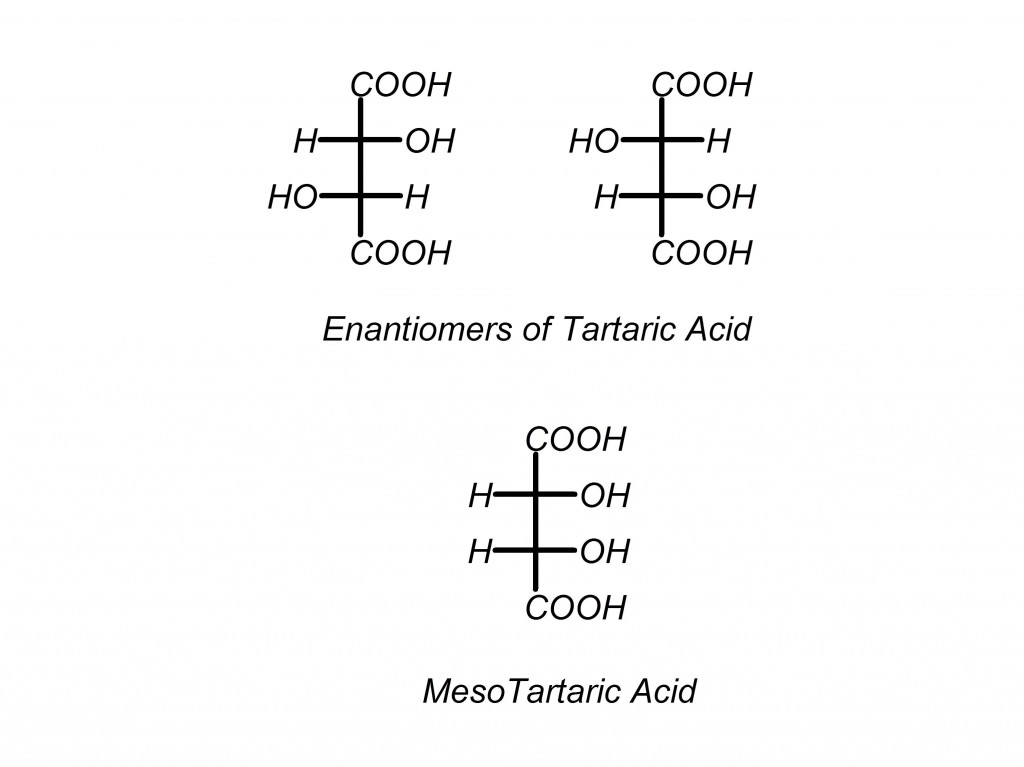
If you use the general rule of 2N (pronounced “2 to the n-th power”) for number of diastereomers, where N is the number of stereocenters, you would be wrong in this case. Here is why: there are three forms of tartaric acid, (R,R), (S,S) and meso tartaric acid. Close examination shows that (R,S) and (S,R) tartaric acid are the same compound, making it meso.
You can also look at it as a Fischer projection, which is an even better way to see what is going on here. (Another reason why it pays to know all of the different types of structural representations)
This is a trick professors will try to pull in first semester organic chemistry, don’t get caught by it. Be sure to look for superimposable mirror images (meso compounds) when doing diastereomer problems on exams. Another place this trick will surface is in NMR problems and calculating the number of unique protons, but that is a post for another time.
For more help with meso compounds, or to take advantage of our testbank or any of our other free resources, please visit us at organic chemistry.