What is a constitutional isomer?
HINT: It is the same thing as a structural isomer and configurational isomer.
Are you wondering if you have a constitutional isomer? There are two questions you need to ask yourself before we can answer this question. The first question: do the compounds have the same molecular formula? If they do not have the same molecular formula, then they are different compounds and not isomers. If they do have the same molecular formula, then they are isomers. The second question: what type of isomer are they? If all of the bonds and atoms of the two compounds you were looking at are in the same order, then they are stereoisomers. (There are all sorts of different types of stereoisomers, but we’ll deal with that later). If they have different connectivity, meaning that the atoms and bonds are in a different order from each other, then you have constitutional isomers.
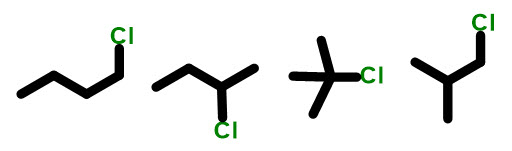
So, to answer the question what are constitutional isomers? They are compounds with the same molecular formula BUT different connectivity. Let’s look at some examples of constitutional isomers. Here is a typical problem you might see on an exam: draw all the constitutional isomers of C4H11Cl.
You can see here that all we are doing is putting different parts in a different arrangement. First, we start with straight chain butane and put the chlorine in two different positions. Next, we start rearranging the carbon backbone, after each change pausing to move the chlorine around. In this example, there is only one carbon backbone change that can take place, so we make that change, move the chlorine and have the right answer. BEWARE: because this is a short chain, chlorine can only be in the 1 or 2 position….there is no 3 or 4 position since we also have to name it to have the lowest number possible.
This is 1-chloro, 2-methylpropane NOT 2-methyl, 3-chloroproane.
Professor Trick: are these compounds constitutional isomers?
The answer is no because they are the same molecule.
Professor Trick: Are these compounds constitutional isomers?
The answer is no because they have different formulas, which makes them different molecules.